What Element Has 15 Electrons
Contents
- i Fundamental Stage two
- 1.1 Pregnant
- two Key Phase 3
- 2.i Significant
- 2.2 Nigh Phosphorus
- ii.2.1 Molecular Structure
- ii.2.two Atomic Structure
- 2.ii.3 Properties
- 3 Key Stage iv
- 3.i Meaning
- iii.two About Phosphorus
- 3.2.1 Molecular Structure
- 3.2.2 Atomic Structure
- 3.two.three Properties
- iii.3 References
- three.3.1 AQA
Key Stage 2
Meaning
Phosphorus is a solid.
Key Stage iii
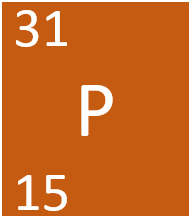
The chemical symbol for Phosphorus.
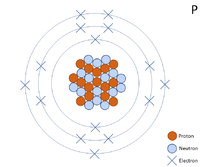
A two dimensional representation of a Phosphorus atom with 15 protons and 16 neutrons in the nucleus and xv electrons orbiting the nucleus.
Meaning
Phosphorus is a Group 5 non-metal chemical element, on the Periodic Table, with an atomic number of 15.
Near Phosphorus
- Phosphorus has the chemical symbol P.
Molecular Structure
- The chemical formula for a Phosphorus molecule is Piv.
Atomic Construction
- Phosphorus has xv protons and 16 neutrons in its nucleus giving it an diminutive number of 15 and a diminutive mass of 31.
- Phosphorus is in Catamenia 3 of the Periodic Tabular array because it has 3 electron shells.
Properties
- Phosphorus is a solid at room temperature.
- Phosphorus reacts rapidly in the presence of Oxygen and it used in matches.
Fundamental Stage 4

The chemical symbol for Phosphorus.

A 2 dimensional representation of the Bohr Model of a Phosphorus-31 isotope with 15 protons and 16 neutrons in the nucleus and two electrons in the first shell, 8 in the second and v in the outer shell.
Meaning
Phosphorus is a Group 5 non-metallic element, on the Periodic Table, with fifteen protons in the nucleus.
About Phosphorus
- Phosphorus has the chemical symbol P.
Molecular Structure
- The chemical formula for a Phosphorus molecule is Pfour.
- Phosphorus forms a covalent bail with 3 other Phosphorus atoms to produce a elementary covalent molecule.
Atomic Structure
- The almost common stable isotope of Phosphorus has 16 neutrons in its nucleus giving it an atomic mass of 31.
- Phosphorus is in Period 3 of the Periodic Table because information technology has 3 electron shells.
- Phosphorus has 5 electrons in its outer beat and needs iii more electrons to get a total outer trounce then it can form 3 bonds with other atoms.
Properties
- Phosphorus is solid at standard temperature and force per unit area.
- Phosphorus is highly reactive in the presence of Oxygen.
References
AQA
- Phosphorus, pages 35, 47, GCSE Chemistry; Pupil Book, Collins, AQA
What Element Has 15 Electrons,
Source: https://keystagewiki.com/index.php/Phosphorus
Posted by: hinkleofue1956.blogspot.com


0 Response to "What Element Has 15 Electrons"
Post a Comment